In a recent study published in the BMC Medicine Journal, researchers conducted a modeling study to evaluate the cost-effectiveness and health impacts of the next-generation vaccines against influenza in Kenya.
 Image Credit: Riccardo Mayer / Shutterstock
Image Credit: Riccardo Mayer / Shutterstock
Background
Influenza is highly prevalent among children below the age of five in Kenya. The recurrent peaks of influenza infections in sub-tropical and tropical regions and the occurrence of infections throughout the year make the choice of vaccine formulation (southern or northern hemisphere vaccine) and the decision of whether or not to vaccinate challenging. This difficulty is exacerbated by the short-lived and strain-specific immunity granted by the current vaccines.
Based on the willingness-to-pay threshold, the current vaccines are not cost-effective since the presence of multiple influenza strains and the inconsistent seasonality of infections necessitate revaccinations every year.
Various next-generation vaccines, such as those using nano-particles and messenger ribonucleic acid (mRNA), have been focusing on conserved proteins in the influenza virus, which might stimulate T-cell responses, reducing the need for annual vaccinations.
Mathematical models involving transmission dynamics, interventions, and control strategies are ideal for evaluating whether these next-generation vaccines could be cost-effective.
About the study
The present study used Kenya’s data on severe acute respiratory illness between 2010 and 2018 and a disease transmission model to evaluate next-generation vaccines with broader cross-protection, longer immunity, and higher efficacy.
Four models were evaluated in the study. The first model, the vaccination model, does not consider the prior vaccination or infection status while examining the level of vaccine-induced immunity. The population is considered susceptible to influenza, vaccinated and susceptible, or vaccinated and recovered from influenza.
The epidemic model (second model) used existing data to identify epidemic periods specific to 11 subtypes. Influenza epidemics were defined based on the proportion of subtype-specific cases with positive test results greater than the weekly average during the study period.
The third model used data on patients hospitalized with severe acute respiratory illness between 2010 and 2018 to determine the epidemiology of influenza, and the model was based on the background force of infection. Finally, the fourth model analyzed the cost-effectiveness of several vaccine scenarios compared to those without vaccines.
Additionally, the health outcomes associated with various vaccine scenarios are calculated based on disability-adjusted life years (DALYs) weighted for mild upper respiratory tract infections, moderate and severe lower respiratory tract infections, and mortality.
The cost-effectiveness was determined based on each DALY’s median incremental cost-effectiveness ratio (ICER) and the median incremental net monetary benefit (INMB). These were used to compare each vaccine and no-vaccine scenario across the ten years. The highest INMB and lowest ICER scenario would be the most cost-effective.
Results
The results suggested that based on the assumed willingness-to-pay threshold and the characteristics of the vaccine, next-generation vaccines could provide cost-effective protection against influenza with a more significant impact than those exhibited by the current vaccines.
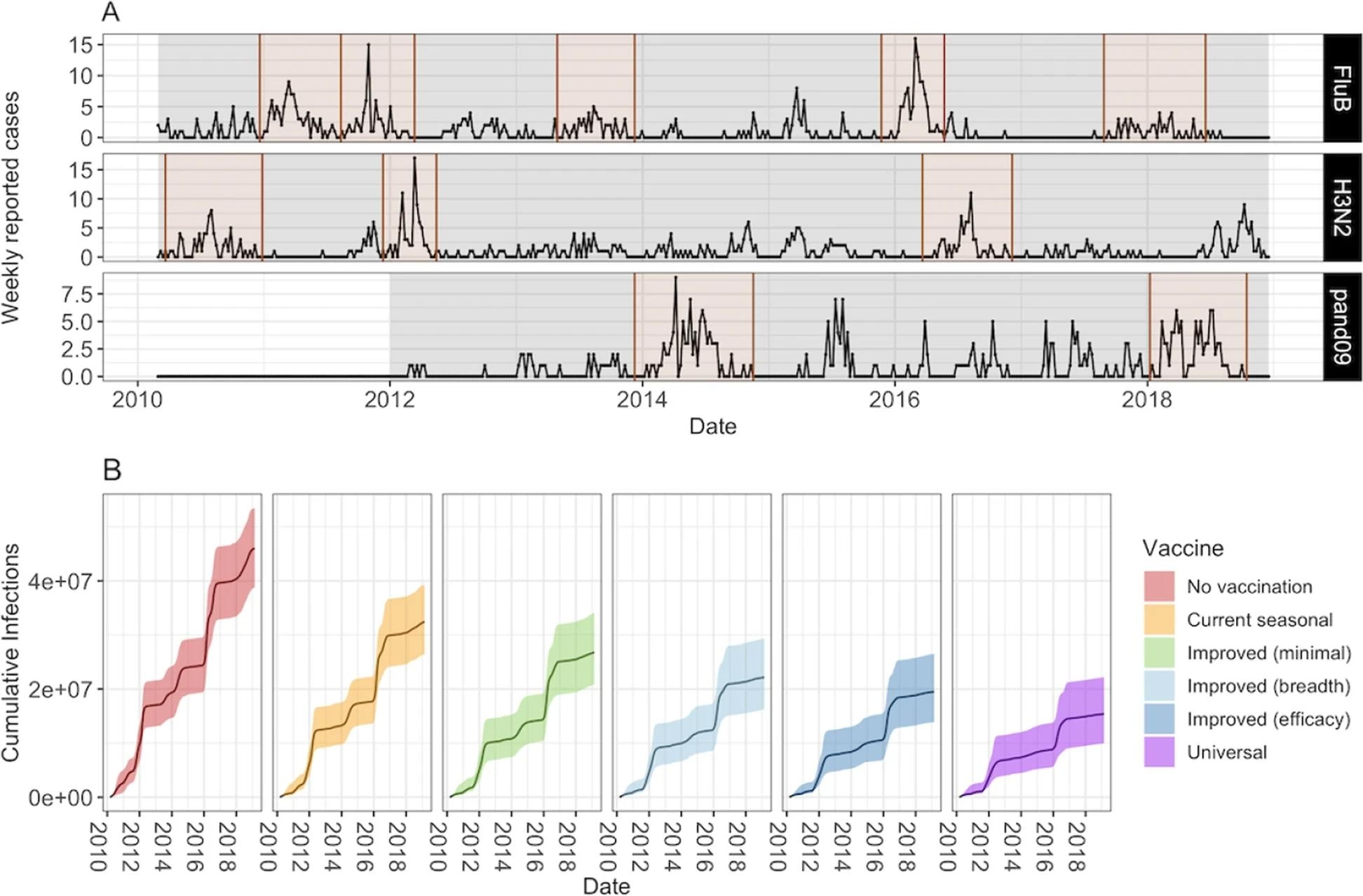 Number of weekly reported cases during epidemic and inter-epidemic time periods. Epidemic periods are highlighted in brown, and periods used to estimate the background force of infection are shown in grey. B Model projections of cumulative number of infections (median and 95% CrI) by vaccine scenario
Number of weekly reported cases during epidemic and inter-epidemic time periods. Epidemic periods are highlighted in brown, and periods used to estimate the background force of infection are shown in grey. B Model projections of cumulative number of infections (median and 95% CrI) by vaccine scenario
The cost-effectiveness is higher than the current vaccines for even incrementally better vaccines that show a slightly longer duration of immunity and greater breadth of protection.
Universal vaccines, which are thought to provide broader and more long-lived immunity, are estimated to avert 66% of influenza infections compared to a no-vaccine scenario. In comparison, the current vaccines are seen to prevent only 29% of influenza infections. In comparison, even minimally improved vaccines prevent 41% of infections.
The ICER values for the current and improved influenza vaccines are 10.55 and 1.60–2.38 times higher compared to universal vaccines, respectively. Universal vaccines also have 2.50 higher INMB values than the improved influenza vaccines at willingness-to-pay thresholds of $623 (United States dollars).
At the willingness-to-pay threshold of $5,738, the threshold at which current influenza vaccines are cost-effective, the universal vaccines have a 4.39 times higher INMB value.
Conclusions
The study provides important evidence to facilitate country-level decision-making about introducing next-generation vaccines. It highlights that next-generation vaccines will likely have a much greater impact and better cost-effective outcomes than currently available vaccines.
Overall, the results suggested that out of the tested next-generation vaccines, universal influenza vaccines present the most cost-effective option for protection against influenza, with a median cost of $5.16 or less for one vaccine dose.
Additionally, the study demonstrated the advantage of using mathematical models and cost-effectiveness thresholds in evaluating whether improved vaccines present viable health intervention options.

 PARENTING TIPS
PARENTING TIPS PREGNANCY
PREGNANCY BABY CARE
BABY CARE TODDLERS
TODDLERS TEENS
TEENS HEALTH CARE
HEALTH CARE ACTIVITIES & CRAFTS
ACTIVITIES & CRAFTS