[ad_1]
In a recent research paper posted to the medRxiv* preprint server, scientists explored the safety of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) BNT162b2 vaccine in children under five years of age.
 Study: Safety of the BNT162b2 mRNA COVID-19 Vaccine in Children below 5 Years (CoVacU5) – an Investigator-Initiated Retrospective Cohort Study. Image Credit: Volodymyr Maksymchuk / Shutterstock
Study: Safety of the BNT162b2 mRNA COVID-19 Vaccine in Children below 5 Years (CoVacU5) – an Investigator-Initiated Retrospective Cohort Study. Image Credit: Volodymyr Maksymchuk / Shutterstock
Background
The efficacy and safety of the coronavirus disease 2019 (COVID-19) messenger ribonucleic acid (mRNA) BNT162b2 vaccine have been evaluated in more than 2,500 adolescents and children aged ≥5 years. Nevertheless, the European Medicines Agency has not yet authorized using COVID-19 vaccinations in children under five years, while investigations testing SARS-CoV-2 vaccines in this age range are underway. Off-label COVID-19 vaccinations in children under five years are lawful in Germany following the acquisition of informed consent from legal guardians/parents. However, it is at the risk of legal guardians, healthcare professionals, or parents.
The SARS-CoV-2 advisory board of the German government announced on February 17, 2022, that in the event of a pandemic, the wellbeing of children must be prioritized, including systematic initiatives to examine potential adverse effects associated with the COVID-19 vaccines. Yet, the safety of COVID-19 vaccines in children under five years is uncertain presently.
About the study
In the current study, the scientists retrospectively assessed the safety of the SARS-CoV-2 BNT162b2 vaccine administered off-label in children aged less than five years in Germany from April 14 to May 9, 2022. The study was named CoVacU5, an investigator-launched retrospective cohort research comprising children under the age of five enrolled for COVID-19 vaccination in ambulatory care centers in Germany.
Inclusion criteria comprised registering in a database for a minimum of one dose of BNT162b2 given before the age of five years and receiving informed consent from a legal representative/parent to partake in the current study’s survey. Eligible subjects were identified using electronic registration systems of 21 ambulatory care institutions and two countrywide layperson-embarked SARS-CoV-2 vaccination campaigns in Germany. Exclusion criteria included duplicate replies with clashing sex, age, height, or weight without specifying whether the children were triplets or twins and respondents who did not have an authentication code as evidence of the invitation.
CoVacU5 documented short-term safety information from one to three shots of three to 10ug BNT162b2 vaccination in children aged zero to less than five years. Co-primary outcomes of the study were the incidences of 11 kinds of symptoms after vaccination with bivariate assessments and regression models controlling for sex, age, height, and weight. General reactions, vaccination-site symptoms, skin changes, fever, any cardiovascular, musculoskeletal, nervous, or pulmonary system, gastrointestinal tract, throat/neck/ear symptoms, psychological issues, or vulnerability to infections were among the symptom categories. In addition, non-SARS-CoV-2 vaccinations used on-label acted as controls via an active-comparator approach.
Results and conclusions
According to the study results, there were 7806 children in this research, indicating a response rate of 41% out of 19,000 enrolled children. Moreover, 338 children were immunized with the first shot of the BNT162b2 vaccine between zero and one year, 1272 between one and two years, and 5629 between two and less than five years.
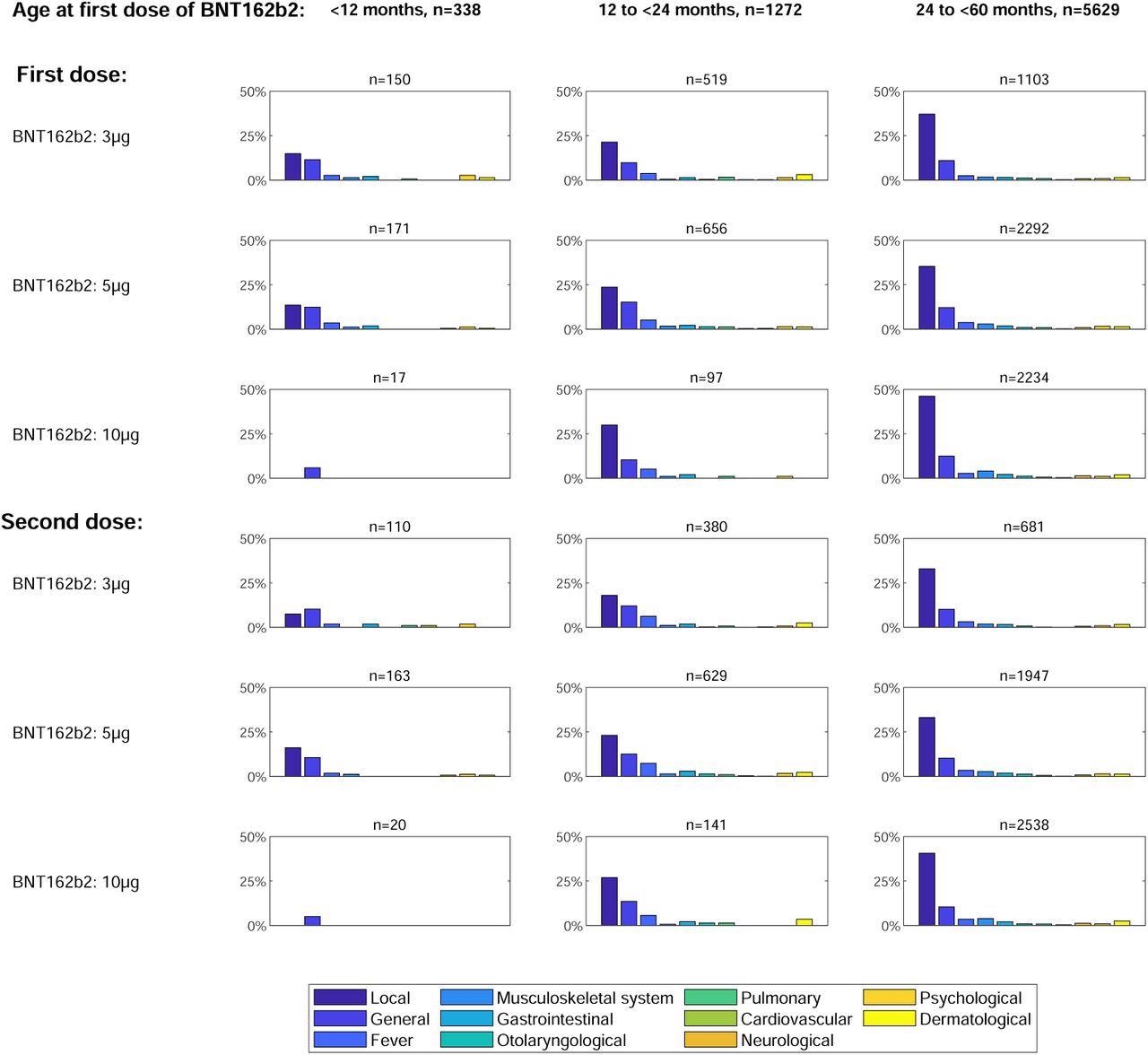
Symptoms reported after BNT162b2 vaccine administration according to age group and dosage. Frequencies of retrospectively reported symptom categories occurring after the first vaccination (upper panel) or the second vaccination (lower panel) with BNT162b2.
A 10 µg BNT162b2 dosage was linked more commonly to injection-site symptoms versus lower doses. Hence, this must be accounted for in upcoming dosage-finding techniques in this age range and carefully evaluated against possible immunogenicity improvements at larger dosages.
The likelihood of any musculoskeletal, injection-site, otolaryngological, or dermatological symptoms was somewhat higher following BNT12b2 versus non-SARS-CoV-2 vaccinations. On the other hand, the BNT162b2 vaccination reduced the likelihood of general reactions and fever. Furthermore, ten symptoms needing hospitalization were described only at BNT162b2 doses greater than 3ug. Besides, no serious adverse events (SAEs) were documented in the children vaccinated with the 3ug BNT162b2 vaccine. Moreover, no deaths were reported in this study.
Conclusions
According to the authors, this was the first study showing that the SARS-CoV-2 BNT162b2 vaccination has a self-documented safety profile equivalent to non-COVID-19 vaccinations in a large group of children under five years. They stated that even after the ongoing phase 1/2/3 trials of BNT162b2 are completed, these results may be valuable in safety considerations for personal decision-making and might supplement data awaited from prospective licensing investigations for expert advice regarding BNT162b2 vaccines in this age range
Overall, the study findings showed that in the current group of children under five years of age, symptoms recorded following the SARS-CoV-2 BNT162b2 vaccination were equivalent to those reported after on-label non-COVID-19 vaccinations. The team mentioned that in addition to the information of the ongoing industry-funded BNT162b2 phase 1 to 3 trials undertaken in this age range, CoVacU5 enhances the available information on COVID-19 mRNA vaccination in children under five years due to the large sample size and multiple doses studied.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Safety of the BNT162b2 mRNA COVID-19 Vaccine in Children below 5 Years in Germany (CoVacU5): An Investigator-initiated Retrospective Cohort Study; Nicole Toepfner, Wolfgang C. G. von Meissner, Christoph Strumann, Denisa Drinka, David Stuppe, Maximilian Jorczyk, Jeanne Moor, Johannes Pueschel, Melanie Liss, Emilie von Poblotzki, Reinhard Berner, Matthias B. Moor, Cho-Ming Chao. medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.05.17.22275005, https://www.medrxiv.org/content/10.1101/2022.05.17.22275005v1
[ad_2]
Original Source Link

 PARENTING TIPS
PARENTING TIPS PREGNANCY
PREGNANCY BABY CARE
BABY CARE TODDLERS
TODDLERS TEENS
TEENS HEALTH CARE
HEALTH CARE ACTIVITIES & CRAFTS
ACTIVITIES & CRAFTS

