In a recent article published in the journal PLOS Medicine, researchers performed a prospective, longitudinal study to estimate the proportion of outpatient consultations resulting in inappropriate antibiotic prescriptions in young children in three low- and middle-income countries (LMICs), Madagascar, Senegal, and Cambodia.
Additionally, they robustly documented disease symptoms and diagnoses in these children while evaluating antibiotic needs during each consultation.
 Study: Inappropriate antibiotic prescribing and its determinants among outpatient children in 3 low- and middle-income countries: A multicentric community-based cohort study. Image Credit: okskaz / Shutterstock
Study: Inappropriate antibiotic prescribing and its determinants among outpatient children in 3 low- and middle-income countries: A multicentric community-based cohort study. Image Credit: okskaz / Shutterstock
Background
The global burden of bacterial diseases is disproportionately large in children, especially in rural settings of LMICs. Antibiotic overuse and inappropriate antibiotic prescribing drive antibiotic resistance, a global public health concern. Thus, characterizing inappropriate antibiotic prescribing among young outpatient children in LMICs is crucial. At present, relevant data from LMICs, where most inappropriate prescribing occurs, at the community level are scarce. Also, the seasonality of inappropriate antibiotic prescribing in LMICs is poorly documented.
About the study
In the present study, researchers recruited all children from the BIRDY cohort who had at least one outpatient consultation during their follow-up, ranging from six to 24 months from baseline. They received systemic antibiotics administered via intramuscular, oral, or intravenous routes. BIRDY, a prospective, long-term, community-based mother-and-child cohort study, took place in urban and rural regions of Madagascar, Senegal, and Cambodia and estimated the incidence of antibiotic-resistant bacterial infections in children below two years.
First, the researchers calculated the main characteristics of the study population in each country, including the number of consultations, diagnoses, antibiotic prescriptions, and inappropriate antibiotic prescriptions.
Next, they categorized all diagnoses made by the physician at each consultation using a syndromic algorithm based on Integrated Management of Childhood Illness (IMCI) guidelines of the World Health Organization (WHO) to determine all infectious diagnoses. Likewise, they used a two-step standardized classification algorithm to determine which health events required antibiotics.
During children consultations, doctors inappropriately prescribed antibiotics to many children, even for health events that did not require antibiotic therapy. Moreover, some children received multiple diagnoses\multiple antibiotic prescriptions during a consultation.
Thus, in this study, the researchers considered the number of children consultations not requiring antibiotics and the number of antibiotics prescribed during consultations but not requiring antibiotics as distinct outcomes.
Further, they used chi2 or exact Fisher tests to compare countries on qualitative and quantitative variables, with the significance threshold of all bilateral tests set at 0.05.
Furthermore, the researchers evaluated risk factors for inappropriate antibiotic prescribing, accounting for the history of the health event, severity score, season, and a child’s age.
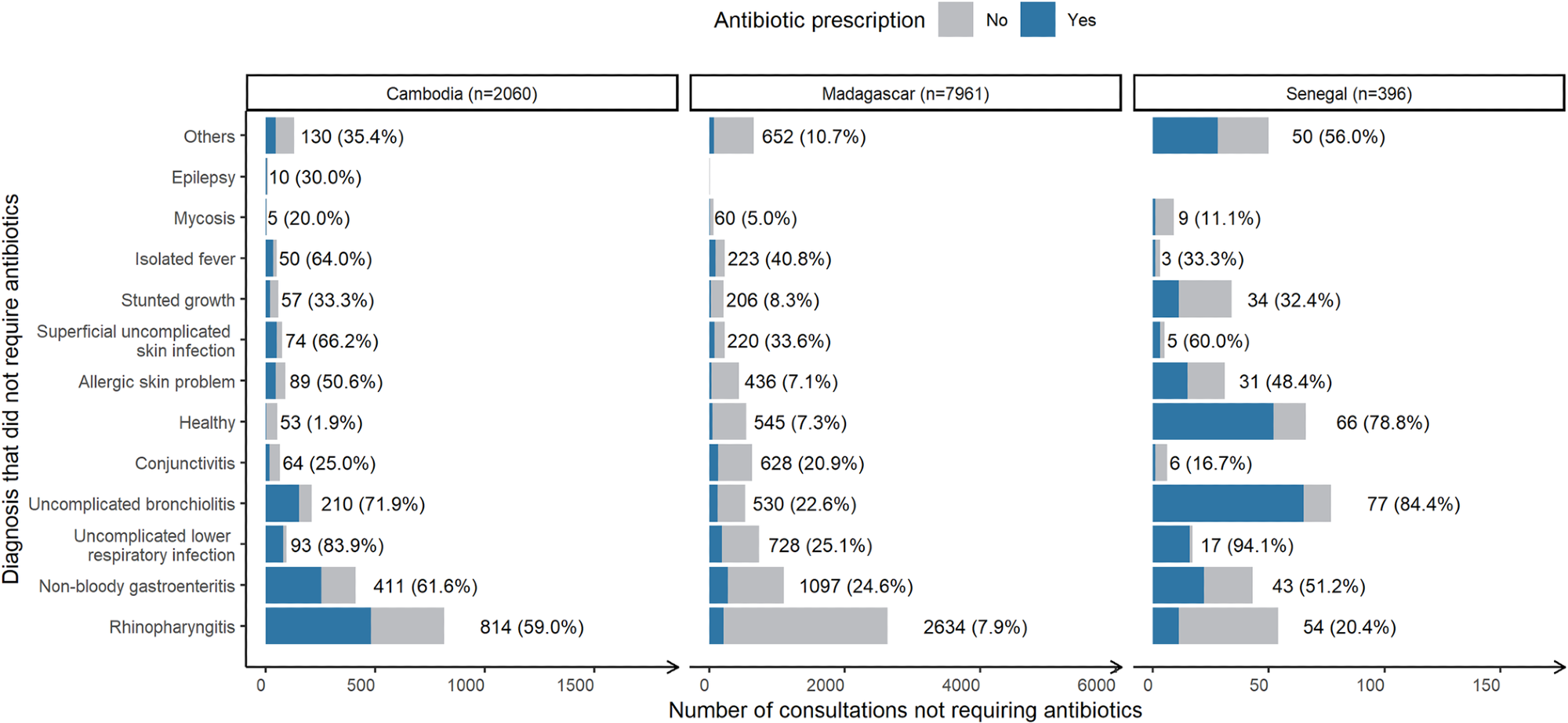 Number of consultations “not requiring antibiotics” stratified by country, associated diagnosis and the share resulting in antibiotic prescription (blue). Across all countries, N = 10,416. *Number of consultations with this diagnosis (percentage resulting in antibiotic prescription).
Number of consultations “not requiring antibiotics” stratified by country, associated diagnosis and the share resulting in antibiotic prescription (blue). Across all countries, N = 10,416. *Number of consultations with this diagnosis (percentage resulting in antibiotic prescription).
Results
Nearly a quarter of outpatient consultations did not require antibiotic therapy; nonetheless, they resulted in an antibiotic prescription. Similarly, three-quarters of all children consultations ending up in antibiotic prescriptions were inappropriate, and approximately two-thirds of those prescribed for young children were unnecessary. While these inappropriate antibiotic prescriptions provided little to no benefit, they inadvertently harmed these young children by contributing to the emergence of antibiotic resistance.
Further, the study results suggested that inappropriate antibiotic prescribing is frequent but highly heterogeneous across countries. Accordingly, while only 16% of consultations not requiring antibiotics resulted in antibiotic prescriptions in Senegal, the figures for Cambodia were much higher, i.e., ~57%.
In this study, researchers documented all disease episodes microbiologically. They noted that 36% of children received antibiotics even when their diagnostic tests detected no pathogens in the stool. In fact, doctors prescribed antibiotics inappropriately for children with gastroenteritis in 24.6%, 51.2%, and 61.6% of cases in Madagascar, Senegal, and Cambodia, respectively.
Likewise, the authors noted that nearly one-fourth of all lower respiratory tract infection(s) consultations, mainly for bronchitis, ended up in inappropriate antibiotic prescriptions in Madagascar but >80% in Senegal and Cambodia. This number, however, remained under 60%, i.e., at 59% and 20.4% for rhinopharyngitis consultations in Cambodia and Senegal, respectively, and was barely at 7.9% for Madagascar.
The authors made another striking observation in Senegal among children consultations reported as healthy yet resulting in antibiotic prescriptions. These diagnoses were quite common, took place at the delivery of a newborn, and 87% occurred in rural areas. This pattern reflects local consultation and prescribing behavior in Senegal, thus, requires careful interpretation.
The most prescribed antibiotic to young children in Madagascar and Cambodia was amoxicillin, which doctors prescribed in 24% and 35.7% of consultations, respectively, and cefixime in Senegal, prescribed in 34.4% of all children consultations. The choice of antibiotics was comparable in Cambodia and Madagascar relative to Senegal.
All-cause antibiotic prescribing ranged between 20% and 60% for all three LMICs evaluated in this study. Neither varying study designs and protocols nor differences in infectious disease burden explain these noticeable intercountry variations in children consultations.
The authors also identified some key determinants and patterns of inappropriate antibiotic prescribing across all three LMICs.
First, they noted that children older than three months than younger infants were at a higher risk of receiving an inappropriate prescription, likely because they are more prone to contracting severe bacterial infections. Second, they noted that clinical severity scores increased the risk of inappropriate antibiotic prescription. In some cases, physicians prescribe inappropriate antibiotics because parents seem overly concerned and express more frequent or severe disease symptoms.
Third, the authors noted that children’s consultations in the rainy vs. dry season more likely resulted in inappropriate antibiotic prescriptions. Fourth, they found that children inhabiting rural areas were at a higher risk of getting inappropriate antibiotic prescriptions, likely because practitioners working in rural settings are usually less qualified. Note that culture, socioeconomic conditions, and healthcare practices (not just the disease burden) largely determine intercountry variations in the quantity and classes of antibiotics sold. However, since the researchers analyzed drug sales data in this study, it provided limited information in this context.
Conclusions
The current study presented a more detailed and accurate picture of inappropriate antibiotic prescribing to children across all three LMICs. Thus, this study highlighted the need for targeted interventions to prevent these practices, such as training prescribing clinicians about valid pediatric clinical management guidelines, e.g., IMCI guidelines, an intervention that dramatically reduced inappropriate antibiotic prescribing in some African countries, e.g., Uganda. Similarly, rapid diagnostic testing might help physicians, especially those practicing in remote areas, understand whether a disease has viral or bacterial etiology and prescribe antibiotics appropriately.
In conclusion, the study results remarkably showed that the decision-making process governing antibiotic prescription is complex; thus, physicians should seek microbiological information and not deviate from established guidelines before prescribing antibiotics to children. Future sociological and qualitative studies might facilitate a better understanding of the perceived necessity of antibiotic prescribing in high-risk populations of LMICs.
Journal reference:
- Inappropriate antibiotic prescribing and its determinants among outpatient children in 3 low- and middle-income countries: A multicentric community-based cohort study, Antoine Ardillon, Lison Ramblière, Elsa Kermorvant-Duchemin, Touch Sok, Andrianirina Zafitsara Zo, Jean-Baptiste Diouf, Pring Long, Siyin Lach, Fatoumata Diene Sarr, Laurence Borand, Felix Cheysson, Jean-Marc Collard, Perlinot Herindrainy, Agathe de Lauzanne, Muriel Vray, Elisabeth Delarocque-Astagneau, Didier Guillemot, Bich-Tram Huynh, On behalf of the BIRDY study group, Plod Medicine 2023, Published: June 6, 2023 DOI: https://doi.org/10.1371/journal.pmed.1004211, https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1004211

 PARENTING TIPS
PARENTING TIPS PREGNANCY
PREGNANCY BABY CARE
BABY CARE TODDLERS
TODDLERS TEENS
TEENS HEALTH CARE
HEALTH CARE ACTIVITIES & CRAFTS
ACTIVITIES & CRAFTS

